An objective of the Milligan University Mission is the practice of sound scholarship, which includes scholarly research. Milligan’s Institutional Review Board (IRB) assures the ethical conduct of all Milligan University research involving human study participants. The purpose of the IRB is to protect the rights and welfare of the human participants who engage in research conducted by Milligan faculty, staff, or students.
The IRB is composed of individuals who are charged with the task of reviewing research involving human participants. All proposed research conducted on behalf of Milligan shall be evaluated by the IRB, which has the authority to approve or disapprove research, or to request modifications prior to approval.
The members of the IRB are guided by the federal requirements defined in Title 45 of the Code of Federal Regulations Part 46 (the Common Rule), which is based on the ethical principles outlined in the Belmont Report. The nature and content of proposed research are evaluated according to Milligan’s IRB policies and procedures as defined in Milligan’s Faculty Handbook. Milligan’s IRB meets regularly during the Fall and Spring semesters. Refer to the meeting schedule for submission deadlines listed below.
| David Gibbons (CHAIR) Ashley Held Kenneth Lang Michael Sweeney | Colleen Weems Nate Wentzel Myra Newman (community member) |
IRB Process Overview
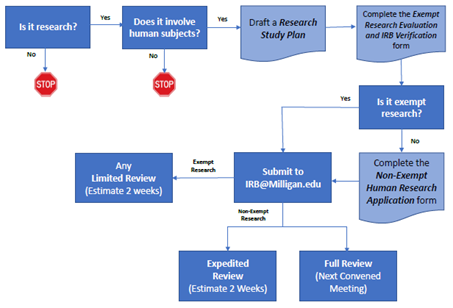
a. Request enrollment in this course by submitting an email request to IRB@Milligan.edu. b. Complete Modules #1, #2, and #3.
a. Determine if the research qualifies for IRB Expedited Review using the Expedited Review Guide (pdf). b. Submit the Non-Exempt Research Application along with the Research Study Plan to the IRB at IRB@Milligan.edu.
a. Any serious or continuing non-compliance with the IRB approved research study plan b. Any serious or continuing non-compliance with the IRB requirements c. Any unanticipated problems involving risks to participants or others related to the research
The IRB Chair will promptly inform all IRB members and the VP for Academic Affairs of any adverse events or outcomes resulting from research conducted at or on behalf of Milligan, in addition to any other required regulatory authorities.
At the completion of the retention period, securely destroy retained research data and specimens. Data and specimens should not be retained longer than the authorized retention period.
A copy of the completed final research report may be retained indefinitely by the Investigator.
2024-25 IRB Monthly Meetings
| Application Deadline | Meeting Date/Time |
| September 10 By 5:00 pm | September 24 11:00 am – 12:00 pm |
| October 8 By 5:00 pm | October 22 11:00 am – 12:00 pm |
| November 5 By 5:00 pm | November 19 11:00 am – 12:00 pm |
| January 14 By 5:00 pm | January 28 11:00 am – 12:00 pm |
| February 11 By 5:00 pm | February 25 11:00 am – 12:00 pm |
| March 11 By 5:00 pm | March 25 11:00 am – 12:00 pm |
| April 8 By 5:00 pm | April 22 11:00 am – 12:00 pm |
